Carpal Tunnel
Sono-Instrument®
(CT)
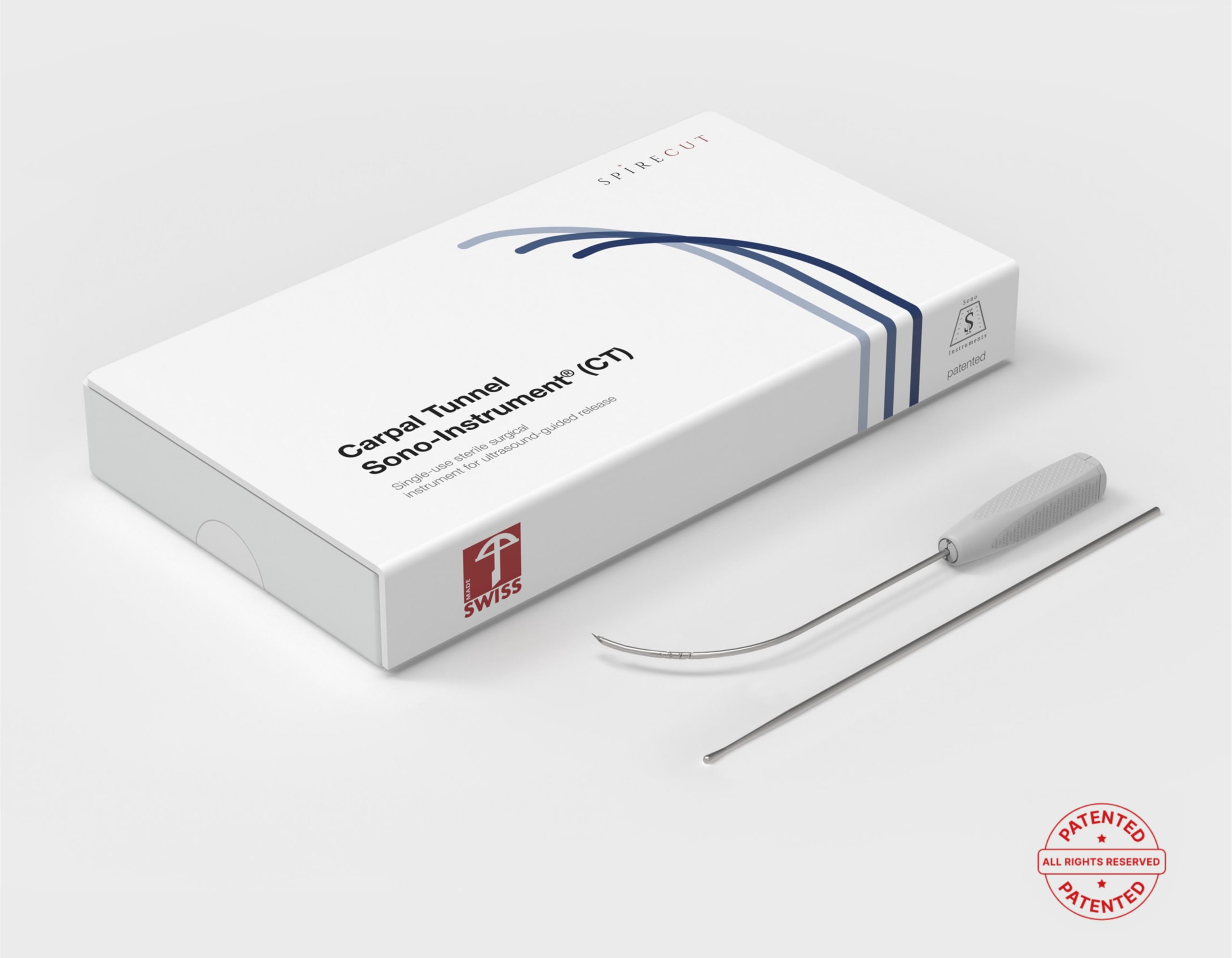
The Carpal Tunnel Sono-Instrument® is a simple and efficient solution to treat carpal tunnel syndrome (percutaneous release of the transverse carpal ligament or flexor retinaculum).
Carpal Tunnel
Sono-Instrument®
(CT)
The Carpal Tunnel Sono-Instrument® is a simple and efficient solution to treat carpal tunnel syndrome (percutaneous release of the transverse carpal ligament or flexor retinaculum).
Carpal Tunnel
Sono-Instrument®
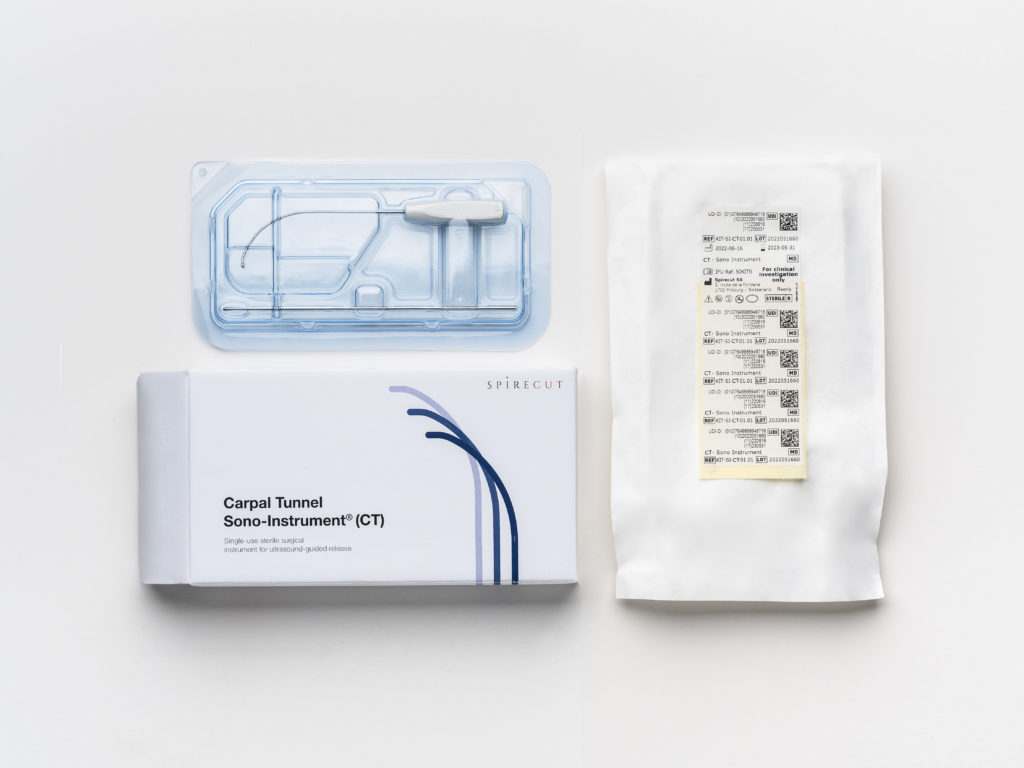
The Sono-Instrument® is packed in a single-use sterile kit, along with a probe for the dissection of the tissues.
Benefits
Safe surgery
The procedure requires ultrasound guidance under local anaesthesia.
Percutaneous surgery
Our technique does not require any skin incision, stitches, or dressing. It does not result in any external blood loss.
Quick surgery
The procedure takes a few minutes to complete, and is performed with minimal instrumentation.
Early return to daily activities
After the procedure, patients can resume their daily activities.
Product details
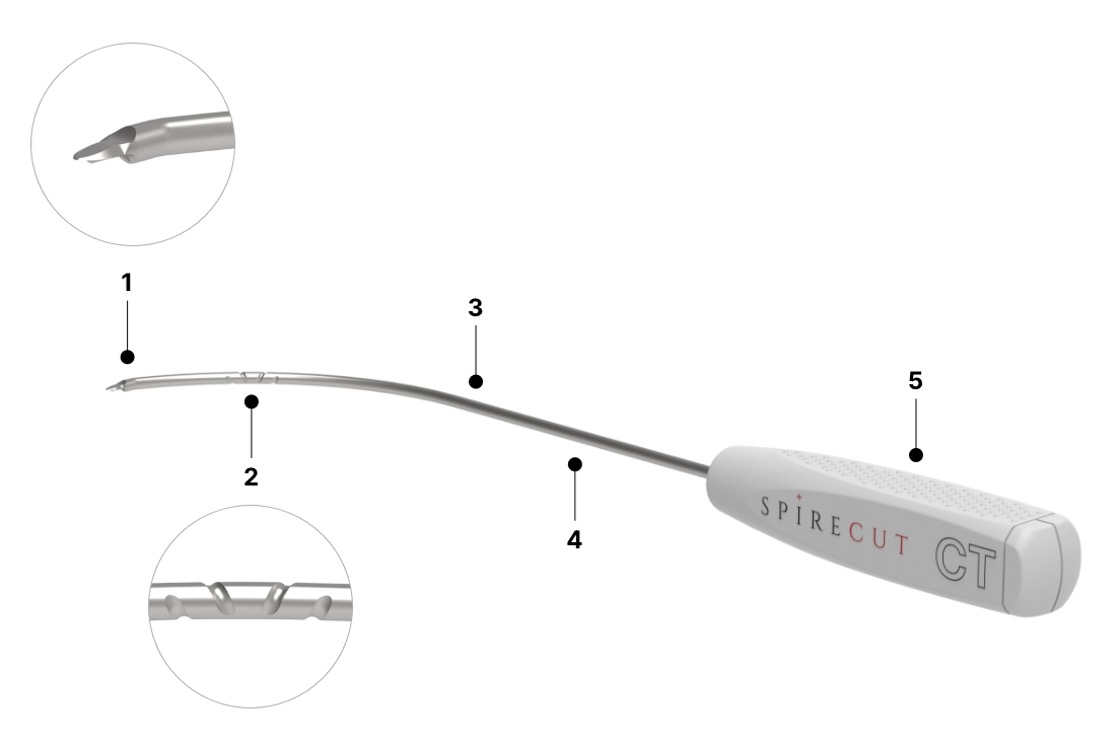
Cutting extremity allowing release in the plane chosen by the surgeon, safe for neighbouring tendons/ nerves/ vessels, with lateral flanges enhancing the echogenicity
Spiral groove facilitating the visualization of the Sono-Instrument® under sonography and providing information about rotatory alignment
Special design of the rod, adapted to the anatomy of the adult carpal tunnel
1.5mm diameter rod, allowing insertion through the skin and fascia by a puncture made by a 14 Gauge IV catheter
Handle, allowing firm non-slip grip of the Sono-Instrument® and the maintain of its orientation throughout the whole procedure of release
Probe
1.5mm diameter smooth probe, facilitating the dissection and allowing at the end of the procedure to check the completeness of the release.

Precautions
The Sono-Instruments® are single-use sterile medical devices intended for mini-invasive hand surgery under sonography. SPIRECUT assumes that professional users have experience and knowledge of standard protocols regarding hand surgery and particularly in percutaneous release procedures under sonography. Furthermore, SPIRECUT declines any responsibility if the users have not followed lab manual-skills training with associated instrumentation prior the procedure. Surgeons are advised to review the instructions for use prior to performing any surgery.
In case of any doubt in identification of the anatomical structures or poor visibility of the Sono-Instruments® during the operation, always convert to open surgery (or stop the surgery).
Surgical technique
The Sono-Instruments® are intended to be used solely in a medical facility, under sterile conditions, by experienced clinicians, usually surgeons, rheumatologists or radiologists, with sufficient knowledge of hand anatomy, hand surgery and sonography. Training during hands-on workshops and/or watching experienced physicians performing the operation is strongly recommended before doing the first clinical cases. Sono-Instruments® are indicated only for patients suffering of either carpal tunnel (SI-CT) or trigger finger/trigger thumb syndrome (SI-TF).
Contra-indications
Do not use SPIRECUT’S Sono-Instruments® :
-
in direct contact with the central circulatory system or central nervous system,
-
in children, dwarf patients or in case of small size hand/carpal tunnel/finger-thumb,
-
if there is a history of a previous infection, as the infection could have caused fibrosis, or as the operation can re-activate the infection, causing delayed healing and/ or severe complications,
-
in case of active or past infections, because the infection may be spread to healthy tissues and made worse by the operation,
-
in conditions that tend to limit the patient’s ability or willingness to understand per or postoperative recommendations during the healing period,
-
for surgeries other than those indicated or at another anatomical location,
-
in case of known allergic reaction to metals,
-
in case of known clinical risks associated to the use of Sono-Instruments® outweighing the expected clinical benefits,
-
in case of anatomical abnormalities affecting the flexor digital sheath, digital nerves/vessels, median nerve, median nerve artery, flexor tendons, wrist bones or in case of local tumors or other conditions increasing the risk of an iatrogenic lesion ; a trigger finger of long evolution, with stiffness of the proximal interphalangeal joint, constitutes a relative contra-indication as it could be better treated by open surgery with removal of a superficialis tendon slip,
-
in case of an alteration of the coagulation with significant risk of per/postoperative bleeding,
-
in case of any contra-indication to anesthesia, whether local, regional or general,
-
in case of insufficient sonographic identification of the operated tissues or insufficient experience of the user in sonography,
-
in case of neuro-vascular structures in the zone of intended release, like may be seen in anatomical variations or vessel dilatation like seen in case of an arterio-venous fistula,
-
in case the physician has insufficient experience in hand surgery and particularly deficient knowledge of hand anatomy, including anatomical variants,
-
in case of tissue adhesions which may potentially compromise the safe and precise introduction of the probe and then of the Sono-Instrument,
-
in case of a previous surgical attempt to treat the condition, for example a persistent disease after previous open, endoscopic or percutaneous procedure, or a recurrence of the condition with persistent or recurrent symptoms,
-
in case of a previous fracture or dislocation in the operated area or any affection causing malalignment or distortion of the local skeleton, due to trauma, arthritis or other causes,
-
for the carpal tunnel syndrome, in case of severe median nerve dysfunction requiring microsurgical epineurotomy, or if the nerve dysfunction has another cause that an idiopathic compression syndrome, like a canalar mass (ganglion, tumor, foreign body, or other space-occupying disesases),
-
in case of the devices are plastically deformed, broken or fall down. If the event happens throughout surgical procedure, remove the instrument and all the fragments immediately.
In general, all other contra-indications mentioned for endoscopic release of carpal tunnel or trigger finger/thumb are valid for percutaneous release using Spirecut’s Sono-Instruments®.
Explore our Trigger Finger Sono-Instrument® (TF)
The Trigger Finger Sono-Instrument® TF is a simple and efficient solution to treat the adult common upper extremity affection, the trigger finger.

